GERBU Adjuvant Introduction Page GERBU Adjuvant Product List
GERBU Adjuvant Manuals

ADJUVANT ADJUVANT MM #3001
For monoclonal antibody production
For highest fusion-yields in Mice. Particularly mild and simple to handle, just mix with antigen and inject. Available in tubes of 0.1ml for processing even the smallest amounts of Antigen without loss, as well as vials of 1ml and 5ml. All components are non-toxic and fully biocompatible.
Suggestion for Use Suggestion for Use
Storage: 4-8°C. Ready-made mixture should be stored frozen. Bring to room temperature prior to use.
Withdrawal: Use a sterile plastic syringe, mix with antigen solution and shake.
Dilution Rate: Standard rate is 1:1 with antigen solution, but other ratios are no problem as long as the recommended volume of adjuvant is used and the maximum injection volume at each site not exceeded.
Diluent: Use water as the diluent. If buffer is necessary, use HEPES, MOPS or glycine. Avoid polyvalent ions such as phosphate or citrate which can provoke coagulation of the positively charged particles. A pH between 5 and 6 has often proved to be more favourable than strictly neutral.
Route of application: Subcoutaneous. Intraperitoneal and intramuscular application not recommended.
No.of injection sites: For optimum effect, more sites are always better.
Maximum injection volume per site: Recommendations are given by various Animal Protection Councils all over the world. [c/f. Lenaars, M. et al (1999) ATLA 27, 79-102. Also: Nicklas, W., Cußler, K., &Hartinger, J., T (1998) TVT, Tierschutzaspekte bei der Immunisierung von Versuchstieren].
Stability of Antigen- Stability of Antigen Adjuvant mix: No restriction, except antigen solution contains polyanionic compounds which could lead to precipitate.
Schedule of Immunization
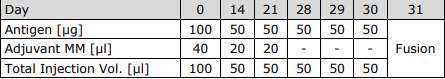
Compatibility
In the host organism the colloidal particles are rapidly rinsed away to the lymphatic system where they perform their action and cannot cause granulomas. All components are proven to be orally and parenterally nontoxic. GMDP has been found to be pyrogenic in doses over 10 µg/kg body mass.
Active Ingredients
The effectivity of Gerbu Adjuvant is based on the synergistic action of the muramyl glycopeptide and solid ultrafiltrable particles of slowly biodegradable lipids added in order to impart the positive electric charges beneficial for optimal efficiency. Equally important are the immunopotentiating, biocompatible emulsifiers and the carefully adjusted synergistic and stabilizing medium which surrounds the nanoparticles.

ADJUVANT ADJUVANT P #3111
For polyclonal antibody production
An Adjuvant for commercial use, developed and proven particularly for the action in small and medium size animals. Powerful potentiator of cellular and humoral immune response. Particularly mild and simple to handle, just mix with antigen and inject. All components are non-toxic and fully biocompatible.
Suggestion for Use Suggestion for Use
Storage: 4-8°C. Ready-made mixture should be stored frozen. Bring to room temperature prior to use.
Withdrawal: Use a sterile plastic syringe, mix with antigen solution and shake.
Dilution Rate: Standard rate is 1:1 with antigen solution (column 3 table), but other ratios are no problem as long as the recommended volume of adjuvant is used and the maximum injection volume at each site not exceeded.
Diluent: Use water as the diluent. If buffer is necessary, use HEPES, MOPS or glycine. Avoid polyvalent ions such as phosphate or citrate which can provoke coagulation of the positively charged particles. A pH between 5 and 6 has often proved to be more favourable than strictly neutral.
Route of application: Subcoutaneous.
No.of injection sites: For optimum effect, more sites are always better. (column 5)
Maximum injection volume per site: Recommendations are given by various Animal Protection Councils all over the world. [c/f. Lenaars, M. et al (1999) ATLA 27, 79-102. Also: Nicklas, W., Cußler, K., &Hartinger, J., T (1998) TVT, Tierschutzaspekte bei der Immunisierung von Versuchstieren]. For FCA the maximal s/c injection volumina established are 1/10 of the s/c volume in column 4.
Stability of Antigen- Stability of Antigen Adjuvant mix: No restriction, except antigen solution contains polyanionic compounds which could lead to precipitate.
Schedule of Immunization
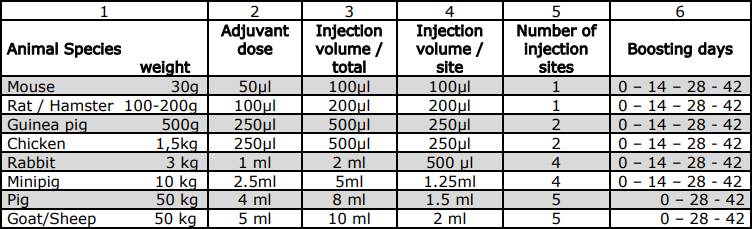
Compatibility
In the host organism the colloidal particles are rapidly rinsed away to the lymphatic system where they perform their action and cannot cause granulomas. All components are proven to be orally and parenterally nontoxic. Generally subcutaneous application works just as well as i/m or i/p and is definitely better tolerated. Chicken especially were observed to stop laying eggs after i/m injections into the breast muscle. GMDP has been found to be pyrogenic in doses over 10 µg/kg body mass.
Active Ingredients
The effectivity of Gerbu Adjuvant is based on the synergistic action of the muramyl glycopeptides and solid ultrafiltrable particles of slowly biodegradable lipids added in order to impart the positive electric charges beneficial for optimal efficiency. Equally important are the immunopotentiating, biocompatible emulsifiers and the carefully adjusted synergistic and stabilizing medium which surrounds the nanoparticles.

ADJUVANT ADJUVANT F #3030
For vaccination of very sensitive larger animals
An Adjuvant for commercial use, highly concentrated for best effect with small injection volumes. Powerful potentiator of cellular and humoral immune response with long term protecting effect. Particularly mild and simple to handle, just mix with antigen and inject. All components are non-toxic and fully biocompatible.
Suggestion for Use Suggestion for Use
Storage: 4-8°C. Ready-made mixture should be stored frozen. Bring to room temperature prior to use.
Withdrawal: Use a sterile plastic syringe, mix with antigen solution and shake.
Dilution Rate: Standard rate is 1:1 with antigen solution (column 3 table), but other ratios are no problem as long as the recommended volume of adjuvant is used and the maximum injection volume at each site not exceeded.
Diluent: Use water as the diluent. If buffer is necessary, use HEPES, MOPS or glycine. Avoid polyvalent ions such as phosphate or citrate which can provoke coagulation of the positively charged particles. A pH between 5 and 6 has often proved to be more favourable than strictly neutral.
Route of application: Subcoutaneous.
No.of injection sites: For optimum effect, more sites are always better. (column 5)
Maximum injection volume per site: Recommendations are given by various Animal Protection Councils all over the world. [c/f. Lenaars, M. et al (1999) ATLA 27, 79-102. Also: Nicklas, W., Cußler, K., &Hartinger, J., T (1998) TVT, Tierschutzaspekte bei der Immunisierung von Versuchstieren]. For FCA the maximal s/c injection volumina established are 1/10 of the s/c volume in column 4.
Stability of Antigen- Stability of Antigen Adjuvant mix: No restriction, except antigen solution contains polyanionic compounds which could lead to precipitate.
Schedule of Immunization
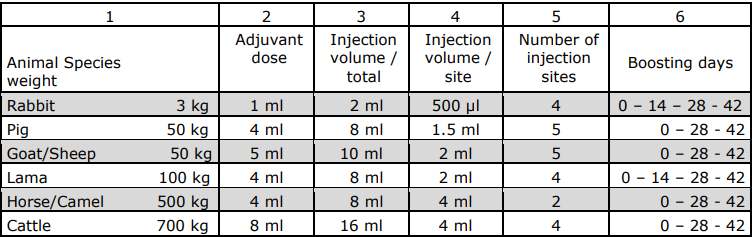
Compatibility
In the host organism the colloidal particles are rapidly rinsed away to the lymphatic system where they perform their action and cannot cause granulomas. All components are proven to be orally and parenterally nontoxic. Generally subcutaneous application works just as well as i/m or i/p and is definitely better tolerated. Chicken especially were observed to stop laying eggs after i/m injections into the breast muscle. GMDP has been found to be pyrogenic in doses over 10 µg/kg body mass.
Active Ingredients
The effectivity of Gerbu Adjuvant is based on the synergistic action of the muramyl glycopeptides and solid ultrafiltrable particles of slowly biodegradable lipids added in order to impart the positive electric charges beneficial for optimal efficiency. Equally important are the immunopotentiating, biocompatible emulsifiers and the carefully adjusted synergistic and stabilizing medium which surrounds the nanoparticles.

ADJUVANT ADJUVANT PluS #3113
For polyclonal antibody production
An Adjuvant for commercial use, developed and proven particularly for the action in small and medium size animals. Powerful potentiator of cellular and humoral immune response. Particularly mild and simple to handle, just mix with antigen and inject. All components are non-toxic, fully biocompatible, and very animal-friendly. High cost-benefit ratio of GERBU Complete Adjuvant (GCA, P) and GERBU Incomplete
Adjuvant (GIA, S)
Suggestion for Use Suggestion for Use
Storage: 4-8°C. Ready-made mixture should be stored frozen. Bring to room temperature prior to use.
Withdrawal: Use a sterile plastic syringe, mix with antigen solution and shake.
Dilution Rate: Standard rate is 1:1 with antigen solution, but other ratios are no problem as long as the recommended volume of adjuvant is used and the maximum injection volume at each site not exceeded.
Diluent: Use water as the diluent. If buffer is necessary, please check before, that the used antigen does not coagulate in the diluent/adjuvant emulsion with applied pH value.
Route of application: Preferably subcutaneous. Intraperitoneal application is possible but not recommended.
No.of injection sites: For optimum effect, more sites are always better (column 5)
Maximum injection volume per site: Recommendations are given by various Animal Protection Councils all over the world. [c/f. Lenaars, M. et al (1999) ATLA 27, 79-102. Also: Nicklas, W., Cußler, K., &Hartinger, J., T (1998) TVT, Tierschutzaspekte bei der Immunisierung von Versuchstieren].
Stability of Antigen- Stability of Antigen Adjuvant mix: Recommending to use Antigen-Adjuvant mixture directly after preparation.
Table of Doses and Application
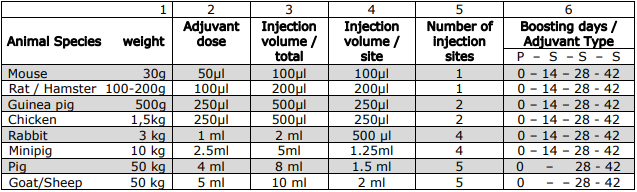
Compatibility
All components are proven to be orally and parenterally nontoxic. Generally subcutaneous application works just as well as i/m or i/p and is definitely better tolerated. Chicken especially were observed to stop laying eggs after i/m injections into the breast muscle. GMDP has been found to be pyrogenic in doses over 10 µg/kg body mass.
Active Ingredients
The effectivity of GERBU Adjuvant is based on the synergistic action of the muramyl glycopeptides and solid ultrafiltrable particles of slowly biodegradable lipids added in order to impart the positive electric charges beneficial for optimal efficiency. Equally important are the immunopotentiating, biocompatible emulsifiers and the carefully adjusted synergistic and stabilizing medium which surrounds the nanoparticles.
*Access to any purchasing capabilities is available only to pre-authorized businesses and their authorized employees, agents and/or contractors who register with VBIOGNOSTICS by submitting a Profile Form and is limited to those users who are authorized to form legally binding contracts under applicable law on behalf of the businesses or organizations they represent.
**All products sold by VBIOGNOSTICS are for laboratory, resale, or manufacturing purposes only. They are not for Human Drug, Food Additive, Clinical or Household use. Only professional laboratory staff should handle these chemicals. Confirmation of your university, institute or corporation will be required for processing orders.**
