BioTechniques News
Aisha Al-Janabi

A novel 3D-cell culture method enables the rapid production of genetically identical ‘lung buds’ for the investigation of respiratory diseases, providing new insights into the pathogenesis of COVID-19.
Recent research from Rockefeller University (NY, USA), led by Charles Rice and Ali Brivanlou, has produced a microchip device capable of producing genetically identical lung buds for the study of infectious respiratory diseases. The device provides a high-throughput option for the investigation of respiratory disease in a highly tuneable experimental setting, which could prove vital in the response to future pandemics.
Examining diseases in authentic human tissue presents the most applicable and clinically relevant mode of discovery; however, there are numerous ethical and practical challenges surrounding the use of these tissues. In particular, the noisy data produced due to the many genetic and phenotypic differences between individual human tissues can bury the key correlations or insights desired from these studies.
For these reasons, while there is a significant amount of postmortem human tissue available for SARS-CoV-2-infected lung tissue, the precise pathway through which this virus initially infects the lungs is not fully understood. During the height of the pandemic, the need for respiratory models that produced clean data was painfully clear, leading Brivanlou to connect with Nobel Laureate Rice to produce such a model. Rice delivered the required biosafety-approved lab and virology experience, while Brivanlou provided the engineering nous and microchip technology required for the project.
 Three advances in stem-cell-derived cardiac models and therapies
Three advances in stem-cell-derived cardiac models and therapies
We’ve collated recent cardiovascular disease research, including developing heart organoids with vascular cells and launching cardiac chips to the International Space Station.
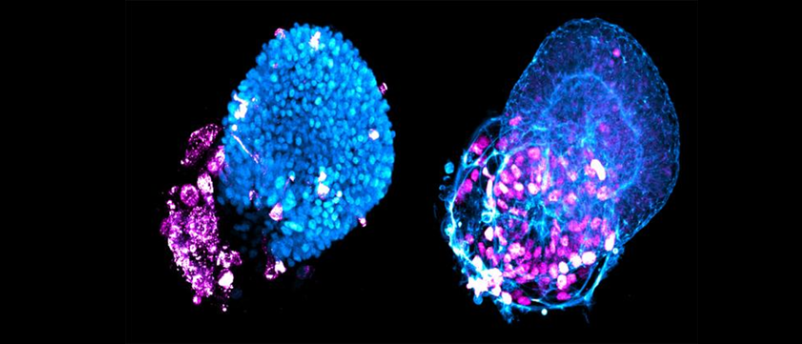
Using human embryonic stem cells (hESCs) hosted in the wells of a microchip array, the team was able to establish a regimen of signaling molecules – an optimal combination of keratinocyte growth factor and bone morphogenetic protein 4 (BMP4) – that encouraged the self-organization of the hESCs into genetically identical lung buds in around 2 weeks. Lung buds are akin to an embryonic precursor of human lungs and are composed of alveolar and airway tissue. This system is able to quickly generate hundreds of these lung buds for infection and morphological studies.
Remarking on the success of this development, Brivanlou commented that the lung buds, “have the exact same DNA signature. That way we don’t have to worry about one patient responding differently from another. Quantification allows us to keep the genetic information constant and measure the key variable—the virus.”
Putting these models to the test, the researchers infected the lung buds at different time points in their formation with a pseudotyped SARS-CoV-2 virus engineered to contain the pNL4-3ΔEnv-nanoluc reporter. This reporter provided the readout for infection using the Nano-Glo Luciferase Assay System to detect Nanoluc Luciferase activity. Transcriptomic analyses were also performed to compare the models with postmortem tissue from COVID-19 patients.
Through these approaches, the team identified that the alveolar cells were more vulnerable to infection than airway cells, which act as the primary barrier to infection in the lungs. The transcriptomic comparison revealed that a marker for active BMP signaling, pSMAD1/5, was upregulated in both the in vitro lung buds and postmortem tissue for infected cells and that the cells expressing pSMAD1/5 were 2.2 times more vulnerable to infection.
The performance of this platform in this study highlights its utility and Brivanlou is keen to emphasize the breadth of its applicability: “We can quickly capitalize on this platform to make a virus visible and develop therapies much faster than we did for COVID. It can be used to screen for drugs, compounds, vaccines, monoclonal antibodies and more directly in human tissue. This technology is ready to confront all kinds of threats that may hit us in the future.”
Next, the team plans to derive models in a similar fashion for further tissues, tipping the kidney, liver and pancreas as their most likely next target.
The post A breath of fresh air for respiratory disease studies appeared first on BioTechniques.
Full BioTechniques Article here
Powered by WPeMatico
